Charged Transport in Polymers
Polymer Nanocomposites
Polymer Upcycling
Charged Transport in Polymers
Conductivity in Nanostructured Precise Polymers
Karen I. Winey (PI)

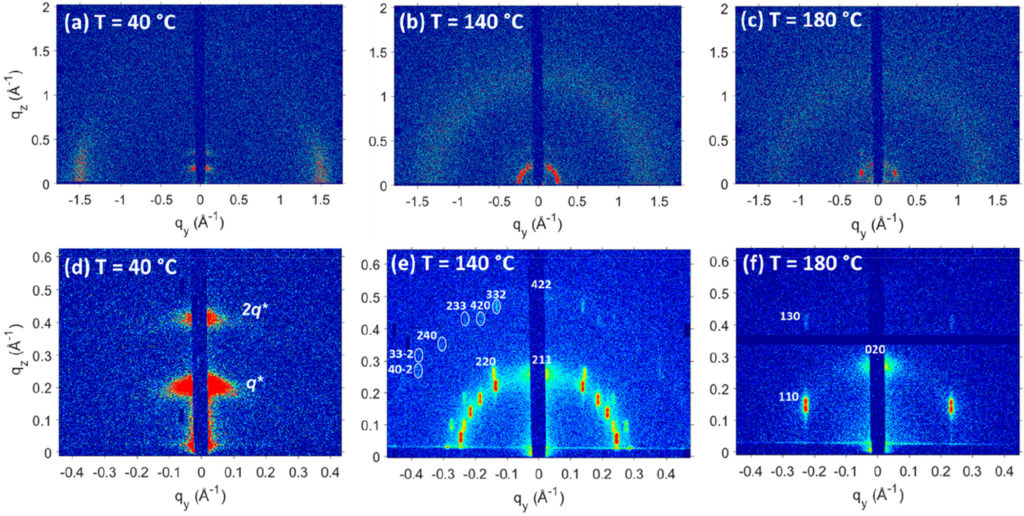
A strong interest in ionomers and other polymers with acid, ionic and polar groups is fueled by their potential ability to selectively transport charged species, which is relevant to batteries, water purification technologies, and fuel cells. The prevailing research directions in the field of solid polymer electrolytes have consolidated around two general classes of homogeneous materials wherein the ions are uniformly distributed throughout the material: polymers mixed with salts and single-ion conductors. The ubiquitous design strategy in these materials systems is based on the understanding that ion conductivity is associated with chain dynamics and ions must be dissociated from their counterion. Unfortunately, these approaches have only limited success in developing suitable polymer-based electrolytes. Winey’s group is exploring an alternative hypothesis, namely that efficient ion conductivity in polymers can be broadly achieved when the ions are sequestered into spatially-continuous nanoscale aggregates and the ionsdissociate from their counterions.
This project builds upon a promising result from Winey’s group wherein proton conductivity of a hydrated precise polyethylene with sulfonic acid groups on exactly every 21st carbon is somewhat higher than a commercial membrane. This precise polyethylene self-assembled into nanoscale layers lined with the acid groups and separated by a crystalline alkyl spacer. The high proton conductivity is evidence that the conducting protons aredecoupled from the motion of the much slower polymer backbones. The proposed project will expand upon this singular finding to establish the merits of Winey’s alternative hypothesis.The proposed research combines conductivity measurements, structural characterization, and molecular dynamics simulations to rigorously interrogate this hypothesis using newnanostructured precise polymers.
The proposed alkyl polyester sulfonates and telechelic oligomers are expected to have crystalline domains that direct the assembly of layered aggregates; these layered morphologies will be aligned in thin films on interdigitated electrodes to explore the fundamentals of conductivity. Random percolated structures in precise polyethylenes with short carbon spacers will also be investigated. Winey will undertake this ambitious project with a set of unfunded collaborators: Prof. Stefan Mecking (Konstanz), Prof. Justin Kennemur (Florida State University), Prof. Paul Nealey (U Chicago), Dr. Amalie Frischknecht (Sandia), and Dr. Mark Stevens (Sandia).
Selected Recent Publications:
- Ordered nanostructures in thin films of precise ion-containing multiblock copolymers.
- Decoupled Cation Transport within Layered Assemblies in Sulfonated and Crystalline Telechelic Polyethylenes.
- Sub-3-nanometer domain spacings of ultrahigh-c multiblock copolymers with pendant ionic groups.
- Superionic Li-ion transport in a single-ion conducting polymer blend electrolyte.
The Role of Local Structure and Dynamics on
Proton and Hydroxide Transport in Ion-Conducting Polymers
Karen I. Winey, University of Pennsylvania (PI)
Amalie L. Frischknecht, Sandia National Laboratories
Michael A. Hickner, Pennsylvania State University
Justin G. Kennemur, Florida State University

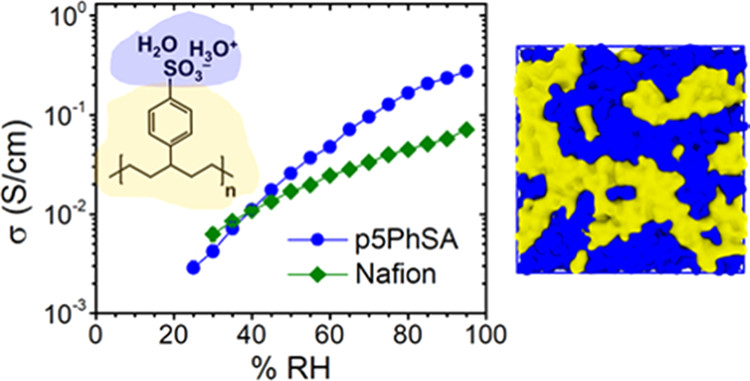
Fuel cells are an increasingly important technology for energy conversion, particularly for transportation. Perfluorosulfonic acid (PFSA) polymers have been used for decades as proton exchange membranes (PEMs) within hydrogen fuel cells, because they have excellent proton transport properties along with high chemical stability. Under high humidity or hydrated conditions, the best-performing PEMs exhibit proton conductivities as high as 0.1 – 0.2 S/cm while still maintaining mechanical stability. However, PFSA membranes have a variety of drawbacks that limit their further development and thus impede progress toward a hydrogen economy, as well as hamper progress in a variety of other technologies (e.g., flow batteries, solar fuels, anion exchange membranes). In particular, fluorine adds expense to the polymer due to both its notoriously dangerous chemistry and its detrimental impact on the environment. Moreover, the fluorine chemistry involved with synthesizing PFSA polymers limits the availability of controlled polymer architectures, such that detailed correlations between polymer microstructure, processing and performance are incomplete. These gaps in understanding significantly impede further development of PEMs, and the understanding of anionic exchange membranes (AEMs) is even further behind. This project endeavors to build a comprehensive and mechanistic understanding of ion transport in hydrated polymers by using a combination of exquisitely controlled chemistry, extensive atomistic molecular dynamics simulations, and a suite of characterization methods that span the critical length and time scales.
Motivated by the critical need for proton and hydroxide conducting membranes for hydrogen technologies, this project will design, synthesize, and study hydrocarbon-based PEMs to mimic the well-accepted salient features of PFSA membranes. The polymers are amorphous, hydrocarbon-based PEMpolymerized via ring opening metathesis polymerization (ROMP) with a phenyl sulfonate pendent group on precisely every fifth carbon. The saturated hydrocarbon backbone provides the conformational flexibility for strong nanophase separation, namely sharp hydrophobic/hydrophilic interfaces with the sulfonic acid groups lining the percolated water channels. A valuable, and as yet untapped, attribute of this new ROMP-based polymer is the exceptional control of the polymer microstructure, particularly of the acid content and of the length of the spacer groups between the saturated backbone and the sulfonic acid group. The nature of the interface between the hydrophobic domains and hydrophilic domains is critically important for controlling proton and hydroxide transport. The attributes of the hydrophobic/hydrophilic interface important for ion transport include the following: the chemical compositionprofiles of the polymer backbone, polymer functional groups and water across the interface; the local roughness of the interface; the interfacial area per functional group; and any correlations between composition and topology at the < 1 nm length scales. In addition, the details of the hydration structuresof the water and ions (protons, hydroxides, sulfonates, quaternary ammoniums) impact proton and hydroxide mobility. Interactions between the ions and the polymer backbone may also affect ion mobility, particularly if the polymer backbone atoms are present at the water domain interfaces. Improving proton and hydroxide conductivity requires better fundamental understanding, particularly with respect to the local structure and dynamics. These ionic domain interfacial structures and hydration interactions will ultimately impact the transport across the entire polymer.
We have assembled a team of four PIs with the combined skills to establish the role of the local structure and dynamics at the hydrophobic/hydrophilic interface on proton and hydroxide transport in these important materials. Our research requires synthetic control and versatility to modify the molecular structure, which is available in Kennemur’s group using ROMP and subsequent functionalization. As recently determined by Frischknecht’s all-atom molecular dynamics simulations and corroborated by Winey’s X-ray scattering, the linear saturated carbon backbones of these polymers have sufficient flexibility to form well-developed percolated water domains. While Frischknecht and Winey have previously combined simulations, electrochemical impedance spectroscopy, and quasielastic neutron scattering to extract new insights about ion and chain dynamics, Hickner brings essential expertise in IR and NMR spectroscopies that will reveal local structure and dynamics in terms of the fundamental role of water in determining the ion transport in these new polymers. Through a highly coordinated effort, our combination of novel polymer synthesis, advanced simulations and comprehensive characterization will provide new understanding of how protons and hydroxide ions move in hydrated polymers.
Selected Recent Publications:
- Fluorine-Free Precise Polymer Electrolyte for Efficient Proton Transport: Experiments and Simulations
- Self-assembled highly ordered acid layers in precisely sulfonated polyethylene produce efficient proton transport
Polymer Nanocomposites
Nanoparticle Interactions and Nanoscale Transport in Polyelectrolyte Brushes
Karen I. Winey and Russell J. Composto

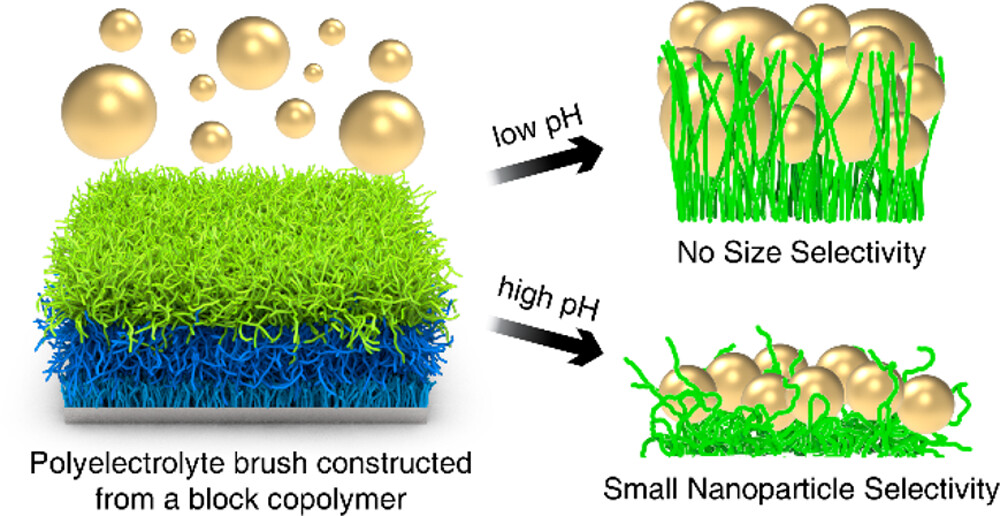
Nanoparticles come in a variety of sizes, shapes, chemistries, and functionalities, ranging from folded proteins, viruses, and quantum dots. Because nanoparticles at interfaces underpin a broad and expanding range of applications including nanoscale filtering and detection, this project will research the motion of individual nanoparticles in complex local environments. Most previous studies of nanoparticle motion have been limited to studies of ensembles of particles or particles in homogeneous environments, and these studies provide a valuable foundation upon which to build. Recent advances in imaging methods and data processing methods enable the study of nanoparticles in heterogeneous environments one particle at a time. Interferometric scattering microscopy provides exceptional spatial and temporal resolution and was originally developed to image biomolecules at interfaces. This project will use this advanced method to study nanoparticle motion at interfaces, particularly interfaces with various functionalities, and under applied electric fields. These studies and fundamental understanding will inform advanced strategies for separating natural or synthetic nanoparticles based on size, shape, or charge by designing interfaces that selectively trap nanoparticles. This new understanding will also provide strategies for producing higher quality nanoparticle products with more uniform characteristics. Differentiating mechanisms of nanoparticle dynamics can also lead to advanced sensing applications.
Winey and Composto will mentor undergraduate researchers, engage with public outreach events across Philadelphia, and partner with a program to bring the science laboratory to underserved high school students.
Bound Layer Exchange in Polymer Nanocomposite Melts
Karen I. Winey and Robert A. Riggleman


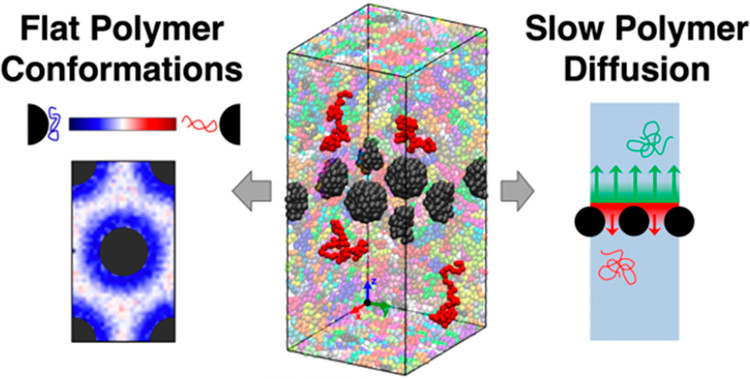
Polymer nanocomposites (PNCs) are an emerging class of materials in a variety of applications including structural materials for infrastructure and membranes for separations. Preventing aggregation of nanoparticles in PNCs frequently requires systems with strong attractions between the polymer and the particles, leading to polymer adsorption to the particle surfaces. This bound layer of polymer on the nanoparticlesexhibits different properties that polymers in the absence of nanoparticles. Overall, properties of the bound polymer layer is critical to the properties and performance of PNCs. For example, bound polymer layers in PNCs can promote uniform nanoparticle dispersion, improve mechanical properties, and tune transport properties. Despite the importance of the bound layer to PNC fabrication and performance, the time-dependentproperties of bound polymers remain largely unknown, especially at the chain-scale. To optimize PNCs properties and effectively design PNCs for advanced applications, additional fundamental studies are necessary to understand and manipulate the bound polymer layer. Meaningful progress requires a multi-pronged effort that combines experiments and simulations to systematically probe the evolution of the bound layer structure and the exchange between bound and unbound polymers in PNCs.
The goal of our collaborative proposed work is to develop and demonstrate a small-angle neutron scattering (SANS) technique to directly probe chain-scale exchange of bound polymers in the matrix (unbound) polymers in PNCs. We will combine this technique with molecular dynamics (MD) simulations to study the effect of annealing temperature and time on the bound polymer layer exchange and to demonstrate control of this exchange by altering the NP-polymer interactions. Achieving these goals will broadly impact the field of polymer science and engineering. Beyond new insights about the chain-scale exchange and properties of bound polymer layers in PNCs, our SANS method can be readily applied to a variety of PNC systems and colloidal solutions. Furthermore, the unique samples fabricated in this study can be used to study the bound or free polymers by various other techniques (e.g. neutron spin echo, NMR).
Selected Recent Publications:
- Effect of surface properties and polymer chain length on polymer adsorption in solution
- “Polymer Conformations and Diffusion through a Monolayer of Confining Nanoparticles”
- “Characterizing the Areal Density and Desorption Kinetics of Physically Adsorbed Polymer in Polymer Nanocomposite Melts”
Polymer Upcycling
Polyolefin Upcycling Through Dehydrogenation & Functionalization
Karen I. Winey, University of Pennsylvania (PI)
Ryan Coughlin, University of Massachusetts, Amherst
Karen I. Goldberg, University of Pennsylvania
Raymond Gorte, University of Pennsylvania
Marisa Kozlowski, University of Pennsylvania
Daeyeon Lee, University of Pennsylvania
John Vohs, University of Pennsylvania

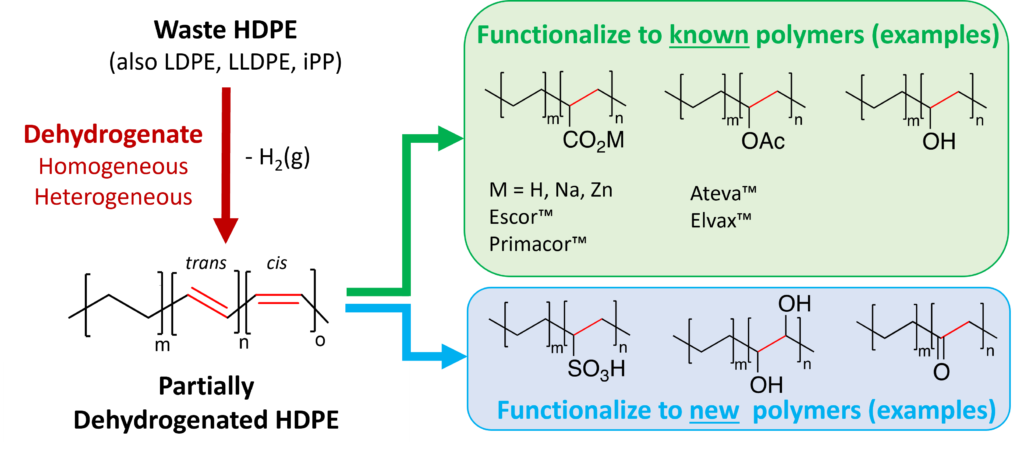
Plastics are inseparable from modern life. Moreover, their light weight, chemical and mechanical durability, selective permeability, and design flexibility allow plastics to mitigate some of the world’s most pressing challenges including reducing food spoilage, preventing the spread of infectious disease, and reducing energy consumption. But the benefits of plastics also come at a cost. Their production, particularly polyethylenes and polypropylenes which are the focus of this work, consumes energy and non-renewable natural resources. After use in the USA, they are overwhelming disposed of in landfills. Our proposed research aims to convert discarded polyethylenes and polypropylenes into specialty polymers via chemical upcycling.
Post-consumer polyolefins (various polyethylenes and polypropylenes) cost ~ $0.20 – $1 per pound, while specialty polyethylene copolymers synthesized by traditional means sell for ~ $5 – $10 per pound and have improved toughness and adhesive properties. To transform specialty polyolefin production from relying on traditional feedstocks to using waste polyolefins as a new, greener feedstock is a significant chemical challenge, because polyolefins have exceptional chemical and thermal stability. Thus, our proposed work endeavors to master the chemical mechanisms of (1) polyolefin dehydrogenation (converts carbon-carbon single bonds to double bonds) and (2) chemical functionalization to convert polyolefins into copolymers. Note that commercially successful polyethylene copolymers are often synthesized with just 1 – 10 mol% of the functional comonomer, indicating that only 0.5 – 5% of the backbone carbon atoms in the waste polyethylene need to be converted to produce more sustainable specialty polyolefins. In addition, several of the new chemical transformations investigated will produce new polymers, for example sulfonated polyethylenes and functionalized polypropylenes.
Our innovative approach to transforming polyolefin waste into functionalized polyolefins has two key attributes. First, polymer molecular weight will be maintained or increased through the transformations by avoiding reactions that induce chain scission. This approach is significantly more energy efficient thanreducing a polymer to monomers, purifying the monomers, and then repolymerizing. Second, we are targeting energy-efficient and environmentally-friendly reactions, so that our foundational work can be readily transformed to practice. While our primary effort will focus on a two-step chemical route, we will also investigate one-step chemical routes to simultaneously dehydrogenate and functionalize polyolefins for additional gains in energy efficiency. Our synthetic efforts will be complimented by extensive polymer property measurements and benchmarking against commercial polymers. Our distinctive strategy for converting waste polyolefins into higher-value functionalized polyolefins is applicable to both single- and mixed-stream polyolefin waste.
Our approach to converting waste commodity polymers into specialty polymers is a viable and valuable step toward a comprehensive effort to reduce polymer waste. Moreover, we anticipate that our foundational work will expand the conversion of waste plastics as a feedstock in the production of an ever-increasing range of high-value plastics.