We are delighted to share our latest article in Macromolecules titled “Polyhalohydrins: Investigating Vicinal Functionalities by Ring-Opening of Epoxides on Polyolefins”, the result of a great collaboration with Bryan Coughlin group at University of Massachusetts Amherst.
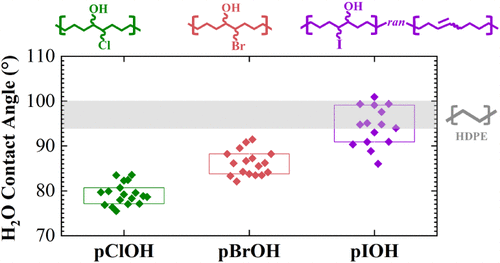
While polymers functionalized with one substituent such as hydroxyl or chloro groups (e.g., EVOH, CPE) are well studied, polymers bearing vicinal functionalities remain largely unexplored. Here, we report the synthesis of three polyhalohydrins (polychlorohydrin, polybromohydrin, and polyiodohydrin) via epoxidation of polycyclooctene (PCOE) followed by epoxide ring-opening with haloacids. Incorporation of vicinal halohydrin groups disrupts crystallinity, and X-ray scattering indicates the presence of dimeric and trimeric associations. Adhesion testing reveals enhanced interfacial performance, with polychlorohydrin exhibiting nearly a threefold increase in lap joint shear strength relative to hydrogenated PCOE, consistent with the increased polarity and surface energy observed across the series.
These findings establish vicinal halohydrin functionalization as a versatile strategy for expanding the chemical modification toolbox of polyolefins, offering a pathway to tune interfacial and macroscopic behaviors such as adhesion through controlled introduction of vicinal, orthogonally accessible functionalities.